Celebrating a long and successful relationship with MDA
The initial Haemoband product was developed in 2005 and one of the first Medical Distribution Companies to commit to representing Haemoband to their hospital and clinic marketplace were MDA in Hong Kong who became our distibutors for Hong Kong and Macau back in 2007.
MDA have been great supporters of the product and today, through their dedicated efforts Heamoband is used across many of the region’s leading hospitals including St. Teresa’s Hospital, Tuen Mun Hospital and North District Hospital, all of which consume over 500 units per annum
Founded in 2004, MDA is registered in Hong Kong and over the past 17 years has grown to be a well-recognized name within the local surgical devices sector with excellent working relationships with all hospitals providing surgery and endoscopy services.
MDA has a strong and experienced team with decades of expertise in sales and marketing, distribution, regulatory affairs, and customer service.
Lunwen ZHANG is Haemoband’s principal contact in the business. Founder and Managing Director of MDA , Adrian – as he is known to his western customers – has over 22 years’ experience in the medical device field. An engineer by training with an expertise in medical technologies he held senior marketing and management posts, for Asia and China markets, with Crown Winner (Hong Kong), NiTi Surgical (Israel) and Frankenman Medical (China) before establishing MDA 2004. The company currently employs a full time team of 9
Commenting on the success of Haemoband in the Hong Kong and Macau markets Adrian says
“From the outset our customers were impressed with how easy the Haemoband system was to use in the Clinic environment and the affordability of this haemorrhoid treatment. As a result the Haemoband has become the first line treatment for our customers. Sales have grown steadily over the years and the performance of the product has been very consistent. MDA are very pleased to have such a quality product in our portfolio.”
INSIGHT INTO THE HONG KONG AND MACAU MARKETS
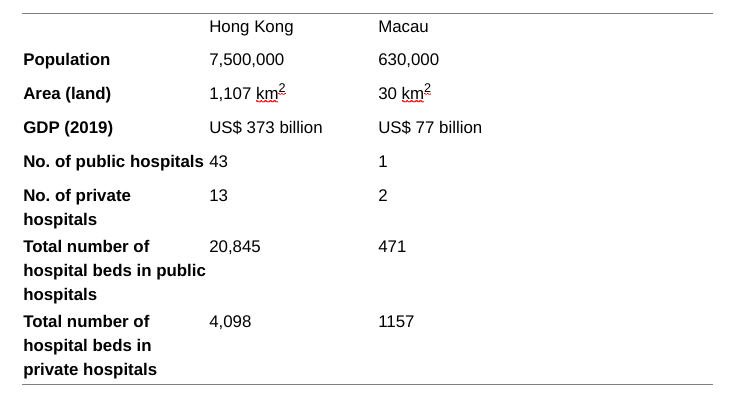
Hong Kong and Macau are the Special Administrative Regions of the People’s Republic of China. Below are some statistics about these 2 regions.
General statistics
 Hong Kong governing bodies and registration
Hong Kong governing bodies and registration
The Department of Health of Hong Kong Special Administrative Region is the governing body of healthcare services to its citizens. All healthcare professionals and organizations are regulated by this department.
In 2004, the Department of Health established Medical Device Division (MDD) which became the regulatory entity for all medical devices imported, sold, and used in Hong Kong. MDD subsequently initiated regulation known as Medical Device Administrative Control System (MDACS), which classifies medical devices into 4 classes.
Currently, the registration of medical devices is still on voluntary base, meaning that for medical devices which are approved by major regulatory agencies (FDA, CE, etc) are allowed to be clinically used in Hong Kong legally. However, public hospitals and major private hospitals often demand the MDACS clearance as essential condition for official purchase or clinical trial. Therefore, MDACS registration has become the quasi–de facto registration requirement for medical devices in Hong Kong.
The registration at MDACS is straight forward. For FDA / CE approved medical devices, the
time of registration takes between 4 – 6 months.
Public hospitals and the Hospital Authority (HA)
The Hospital Authority is the statutory body established under the Hospital Authority Ordinance in 1990. It is responsible for managing Hong Kong’s public hospitals.
HA, which accounts for 85% of major medical treatments in Hong Kong, has the Head Office under which hospitals are grouped into 7 clusters by geography:
Private hospitals
The role of private hospitals is to be complementary to public hospitals, which provide most complex treatments, such as cancer, infectious diseases, organ transplants and mental illness. Due to the exorbitant costs of ICU and adjuvant therapies, these treatments remain largely in the hand of public hospitals, unless the patients are very wealthy or have very generous insurance coverage.
As the HA waiting list, which is often very long, is reserved for complex and urgent operations, private hospitals play a major role in benign diseases treatment, such as benign proctological diseases, hernia, cosmetic surgery, and ophthalmology.
The private hospitals are likely to gain higher market share after the government enforces “universal medical insurance” for all citizens. But this process is long.
Healthcare reimbursement and payment from hospitals
In public hospitals, all, except certain expensive drugs and devices, are paid by the HA budgets. In-patient fee is HK$120 / day.
In private hospitals, patients either pay the service out of their own pockets or with private insurance.
Cash flow of most hospitals is very healthy. Public hospitals can usually settle payments around 45 days, and private hospitals in 60 days, unless stipulated otherwise in special purchase contracts.
Tender and procurement in HA hospitals
Generally, the purchases by HA hospitals can be divided into following hierarchy, according to values of purchase:
- Below $50,000: Direct purchase
- Between $50,000 and $100,000: Invitation to quotes is sent to a selected number of known suppliers for comparison.
- Above $100,000: Public tender is issued. All qualified suppliers can bid.
HA’s tenders can be divided into 2 main types:
- Exclusive contract: awarded to one exclusive supplier for single hospital to ALL public hospitals.
- Qualified suppliers: Awarded to qualified multiple suppliers for the same items at agreed prices over the contract period.
In either case, HA only gives an estimate demand quantity without guarantee to reach it.
MDA frequently participates and wins the tenders of both types.











